 PDF) Flecainide–metoprolol combination reduces atrial fibrillation clinical recurrences and improves tolerability at 1-year follow-up in persistent symptomatic atrial fibrillation
PDF) Flecainide–metoprolol combination reduces atrial fibrillation clinical recurrences and improves tolerability at 1-year follow-up in persistent symptomatic atrial fibrillationWarning: The NCBI website requires JavaScript to operate. 20 years in the preparation: the phlecainide is safe and effective for the management of the atrial fibrillationEtienne Aliot1Département de Cardiologie, CHU de Nancy, Hôpital de Brabois, rue du Morvan, 54511 Vandoeuvre-lès-Nancy Caredex, FranceAlessandro Capuccijette Vincenz Hospital, Paderborn, GermanyJuan Tamargo5Department of Pharmacology, Faculty of Medicine, Complutense University, Madrid, SpainAtrial fever is the most common For the past 25 years, phlecainide has been widely used worldwide, and its ability to reduce AF symptoms and provide long-term restoration of sinus rhythm (SR) has been well documented. The highest mortality observed in patients treated with phlecainide in the Cardiac Arritmia (CAST) study, published in 1991, still discourages many clinicians from using phlecainide, denying many new AF patients a valuable treatment option. There is currently a body of evidence that clearly shows that phlecainide has a favorable safety profile in patients with AF without significant left ventricular disease or coronary heart disease. As a result of this evidence, phlecainide is now recommended as one of the first-line treatment options to restore and maintain SR in patients with AF under current treatment guidelines. The aim of this article is to review the literature on pharmacological characteristics, safety and efficacy of phlecainide, and to place this medication in the context of current therapeutic management strategies for FSA. IntroductionAtrial fibrillation (AF)—a supraventricular tachycardia with rapid and uncoordinated atrial activation and an irregular ventricular rate of heat rhythm, often fast—is increasing, to a extent that cannot be fully accounted for by factors such as an ageing population or a growing prevalence of cardiovascular diseases. Current guidelines for the treatment and management of AF recommend heart rate or rhythm control, as well as conmbotic. The decision on the strategy to be implemented depends on a number of factors, including the pattern of presentation and the presence or lack of underlying conditions. The latest algorithms initially recommend controlling the ventricular rate, based primarily on randomized test results that found no mortality or morbidity advantage of any strategy. However, the same guidelines indicate that prior to choosing the control of long-term rates, the future effects of permanent FAs should be considered. It is important to ensure that a window of opportunity to maintain the sinus rhythm (SR) is not overlooked early in the course of AF management. In a recent position paper it was proposed that some patients with AF, which selects the MR maintenance strategy, may benefit from the previous cardioversion. This was driven by the growing recognition of the importance of structural changes that precede the first documented episode of the AF. Only additional studies will provide the solid data necessary to test this hypothesis. Atrial fibrillation load The prevalence of AF increases with age; it is estimated that 70% of AF patients are between 65 and 85 years old (average: 75 years). The impact of AF on morbidity and mortality has been carefully documented. The 1-year follow-up data from the Euro Heart Survey for 80% of the 5333 participants found that in patients with permanent FA, the mortality rate was 8.2%. In addition, the mortality rate in patients with first-detection AF was 5.7 per cent. A strong association (P untreated and/or previously undetected episodes of AF induce electrophysiological and structural changes to the heart muscle, making the restoration of SR increasingly difficult. This vicious circle that contributes to the continuum of the AF is now described as "AF engenders AF". In long-term follow-up studies, significant proportions of patients with paroxysmal (intermittent) FA progressed to persistent (chronic), Hobbs et al. showed that electrical remodeling in AF can be reversed in some patients if SR is maintained from an early stage, suggesting that rapid recognition and management of AF is critical. Objectives of this review Flecainide has been available in Europe since 1982. The results of the heart arrhythmia suppression trial (CAST), published in 1991, showed a higher mortality in patients who survived the myocardial infarction (MI), and caused the sales of class IC antiarrhythmic drugs (AADs) to fall dramatically (by 75%) and there has been a significant decrease since 1995 in the prescription of class III AADs. This review aims to examine the 27 years of accumulated data on the safety and effectiveness of phlecainide, and place the medication in its therapeutic context. It also analyses the pharmacological characteristics of phlecainide that are intended to prevent long-term structural and electrophysiological remodeling, as well as the maintenance of SR (Data on File. 11th Flecainide PSUR: Meda Pharma GmbH " Co KG, 19 August 2008). Atrial fibrillation management Therapeutic options Speed rate control Options to restore and maintain the SR, or control the ventricular rate while allowing the AF to persist, are not mutually exclusive. In the AFFIRM study, a pace control strategy did not show better survival of a speed control strategy in patients with AF, although long-term SR control and anticoagulation therapy were associated with a lower risk of death. Several studies, including large randomized controlled trials, PIAF, STAF, RACE, AF-CHF, HOT coffee and AFFIRM, have shown that in older patients with minimal symptoms, rhythm control using AADs is not associated with better mortality, morbidity or QOL scores, compared to rate control. In fact, the AFFIRM study data suggest that, in older patients with convivial heart disease, the adverse effects of AAD can overcome the benefits of SR restoration. A meta-analysis of STCE, STAF, PIAF, HOT CAFÉ and AFFIRM trials confirmed that in patients with persistent AF or AF that are likely to be recurrent, ventricular speed control with anticoagulation therapy was equivalent to a rhythm control strategy in the prevention of clinical results. Over time, AF-induced structural and electrophysiological remodeling can lead to heart failure and intractable AF. The growing prevalence of AF in a aged population, along with conditions of slow progress such as hypertension, coronary disease (CAD), obesity and heart failure, suggests that AF itself can be the culmination of a prolonged process. From this perspective, the first detected episode of AF is an important opportunity to prevent disease progression. Test-based treatment guidelines The 2006 CAC/AHA/ESC guidelines on AF that are shown in the chart outline a consensus on treatment in a comprehensive manner, clearly delineating the appropriate options available for each of the different groups of patients with PF found. The availability of new data from recent clinical trials led to an update of the European guidelines of ESC to include dronedarone as a recommended first-line treatment option for the maintenance of the SR in patients with paroxysmal and persistent IC, except those patents with congestive heart failure of class III/IV of the New York Heart Association or unstable congestive heart failure class NYHA II. Test-based treatment guide recommendations for (A) maintenance of the newly discovered sinus rhythm (A) after cardioversion (adapted from Fuster et al.). *, Update of recommendations of the European Society of Cardiology; AF, atrial fibrillation; HF, heart failure; LVH, left ventricular hypertrophy. Focus on phlecainide Phlecainide philacology Flecainide has local anesthetic effects and belongs to the 1C AAD class that block the sodium channels, which delays driving through the heart. It increases selectively the refractority of the anterograted and retrograde accessory pathways. Flecainide action in the heart prolongs the PR interval and expands the QRS complex. The effect in the JT interval is insignificant as phlecainide does not lengthen ventricular repolarization. ,Pharmacokinetics of flecainide The oral administration of phlecainide results in extensive absorption (bioavailability: 90-95%). Phlecainide does not appear to be subject to significant first-step liver metabolism; a daily dose of 200 to 500 mg produced plasma concentrations within the therapeutic range of 200 to 1000 μg/L (the maximum daily dose is 300 mg)., Half of life of elimination is 12 to 27 h. Phlecainide suffers extensive liver biotransformation through P450 CYP2D6 cytochrome; inactive metabolites are excreted mainly (85%) in the urine. Antiarrhythmic Effect In similar concentrations (half maximum inhibitory concentration [IC50]: 1–2 μM), phlecainide blocks the rapid heart current to the Na+ (INa) interior and the rapid component of the late K+ retifier current (IKr).– In higher concentrations (IC50: 19 μM), phlecainide also inhibits late Na+ current and other K+ heart channels. Flecainide has a high affinity for the open-state Na+ channels and significantly delays the time of recovery of the Na+ channels during diastole (τ √≥ 10 s). Thus it has been classified as AAD class 1C., –Flecainide prolongs the potential duration of action (APD) in ventricular and atrial muscle fibers, but shortens the APD in Purkinje fibers, a consistent effect with Na+ channel block. In human atria, phlecainide only increases APD and refractority in cells with a long plateau. However, due to the fact that phlecainide exhibits a very slow kinetic and without joining the Na+ channels during the diastole, it prolongs refractory to a greater extent than the APD (i.e. post-repolarization refractority), decreases excitability and slows intracardiac conduction, even at normal heart values, in all heart tissues. Clinically, this effect appears to be more important at the headphone level. At ventricular level it can cause an increase in the stimulation threshold in patients with artificial pacemakers. Atrial fibrillation conversion mechanism In multi-species superfluous atrial preparations, including dogs and humans driven at fast speeds, phlecainide reduces APD shortening, producing atrial refractory-dependent extension. Flecainide also removes APD headset from heart rate changes in anesthetized dogs, which leads to prolongation dependent on the atrial refractority rate, which may be important to delete the FA. In experimental canine models of AF, the phlecainide finishes the AF, causing an increase dependent on the tachycardia in the refractory period and effective wavelength atrial, reducing the number of re-entrant circuits, so that the arrhythmia can no longer sustain itself., However, in the instrumented goats with multiple atrial electrodes, the progressive cardioversion of FA sustained induced by phlecain The effects on the remodeling of the atrial fibrillation are known to induce significant electrophysiological alterations in the atrial myocytes and cause significant structural changes (structural conformation) in the atrial tissue. – Several molecular alterations related to AF in the cellular and subcellular plane are due to the activation of different signal transduction systems. These molecular pathways are involved in the regulation of genetic expression, cell proliferation, hypertrophy, fibrosis and cell death. Histologically, the fibrillating tissue shows signs of hibernating myocardial, with evidence of ischemic or metabolic lesion of the tissue, for example, disintegration of contropic filaments (myolisis), glucogen accumulation and mitochondrial inflammation. Studies have clearly demonstrated the importance of oxidative stress for the occurrence of such changes in the AF., The increase in the frequency of depolarization during an episode of AF causes a transient increase in the Na+ entry in the atrial myocytes. It is believed that the cytolic accumulation Na+ worsens myocardial injury, mainly as a result of the increase in the Ca2+ entry through the Na+-Ca2+ sarcolemmal exchanger. Curiously, Iwai et al. reported that the cytolic overload Na+ can directly alter the mitochondrial function by depolarizing its internal membrane and reducing the oxidative phosphorylation rate. Therefore, the inhibition of the Na+ channels by phlecainide during the rapid atrial activation should attenuate the excess of cellular accumulation Ca2+ and reduce oxidative stress (Figure ). Importantly, Na+ enters through the Na+ fast-entry current and not the slow-inactivation component of the Na+ current, known as the latest INa (INaL), which flows during the plateau of the cardiac action potential and extends the QT interval. INaL is more sensitive to phlecainide than INa, such that the abnormal entry of Na+ through INaL can be inhibited to drug concentrations that almost have no effect on INa peak. The inhibition of Na+ channels by flecainide during the rapid activation of the headphone attenuates excessive cellular accumulation Ca2+ and reduces oxidative stress. ICa,L, L-current type of calcium; ROS, reactive oxygen species; NFκB, nuclear-κB factor.Preliminary observations support the beneficial effect of phlecainide in the fibrillating human atrial tissue. In an organotypical model of human atrial tissue, oxidative stress markers induced by attenuated phlecainide stimulator and abolished the expression of hypertrophic kinases and inflammatory adhesion molecules. Thus, phlecainide appears to be beneficial to the myocardial lesion induced by the AF and atrial dysfunction. Electrophysiological properties Electrophysiological studies in patients with cardiac arrhythmias show that phlecainide prolongs the right atrial conduction time (AP interval), auriculoventricular node (AV interval) and His-Purkinje (HV interval)., In patients with dual AV nodal pathway, phlecainide selectively prolongs retrograde refractority of the fast track., In patients with accessory AV tracks, phlecainide slows down the drive and increases the refractority of the anterograted and retrograde pathways, but its effects are pronounced more on the retrograded pathway, often causing a complete blockage of regress. Phlecainide produces a dose-dependent decrease in intracardial conduction, but its effects on intraatrial and AV nodal conduction are less pronounced than those of Su-Purkinje conduction and ventricular activation., – Extend PR intervals (17-29%) and QT (4-11%) and QRS complex (11-27%). Most of the QT prolongation is due to an expansion of the QRS complex, so that the JT interval and the QT interval corrected by the types (QTc) are not changed or increased slightly (3–8%). Phlecainide also prolongs atrial, nodal and ventricular refracture, but its effects on refractority are less pronounced than its effects on intracardial conduction. – Flecainide also increases the endocardial stimulation threshold; therefore, it may be necessary for pacemaker-dependent patients to reschedule their pacemakers. Phlecainide does not affect the breast rate, although bradycardia and tachycardia have been reported occasionally., phlecainide increases the recovery time of corrected sinus nodes and the synatrial conduction time (SA) in patients with sinusal node dysfunction. Class 1C AADs, including phlecainide, can cause supraventricular proarrhythmia during AF through a regulatory effect on atrial fibrillatory activity, leading to a slow trial flow typically at a speed of 200 ap. Flecainide does not decrease the conduction of AV and, as a result, a ratio of 1:1 of AV conduction at a high ventricular rate may occur. This is associated with aberrant conduction and a strange QRS morphology caused by exaggerated intraventricular conduction delays. Auriculoventricular nodal block drugs can be used to prevent conduction 1:1 and patients should be instructed to stop exercise when using AF, the conversion of atrial fibrillation to leak is considered proarrhythmia. This effect can be useful as 1C ablation disappears while phlecainide continues invariably leads to control of AF symptoms. In addition, the danger of this type of proarrhymia is eliminated after the ablation of the right atrial isthm. Class 1C ventricular proarrhythmia is manifested as a broad sinusoidal monomorphic QRS tachycardia or as a polymorphic ventricular tachycardia or fibrillation. Factors associated with the risk of ventricular proarrhythmia include a decrease in left ventricular function (LV), ventricular scar tissue, excessive dosage and/or rapid dose increases. Premonitory signs of surface electrocardiogram (GEC) include excessive increases in QRS duration. Late proarrhythmia is the most important threat to patients treated with AADs, especially those with supervenient ischemia or electrolytic alterations. For class 1C medications, CAST has shown that proarrhythmia does not occur exclusively early after the initiation of therapy, but may be ongoing throughout the follow-up. What factors are involved in late proarrhythmia, outside the hospital or sudden death during drug therapy 1C is not clear. Several factors were involved in CAST: the late development of ischaemia, congestive heart failure and the accumulation of drugs at toxic levels. All these conditions dynamically promote the blocking of Na+ channels by class I drugs. Increases in heart rate, which occur during daily life, can set the stage for late proarrhythmia. For example, in patients with reduced LV function, during exercise there may be (sub) acute worsening of congestive heart failure, possibly due to dependence on class I drugs. CAST, results have associated phlecainide with debilitating side effects and higher mortality compared to other treatment options. It is very likely, however, that the highest mortality observed was due to a higher incidence of ventricular fibrillation in this population (the so-called proarritmic effect). As a result, phlecainide is not recommended for use in patients with depressed ventricular function or CAD. Potential for hemodynamic effects Flecainide exerts an inotropic negative effect that can be related to the reduction of the Na+ input with subsequent reduction Ca2+ in myocardial cells. In addition, it blocks the intracellular interaction between Ca2+ and the ryanodine receptor; new data presents phlecainide as a novel strategy to prevent the Ca2+ diastolic waves resulting in triggered arrhythmias. In isolated ventricular myocytes of a polymorphic ventricular tachycardia mouse model, phlecainide inhibited the channels of cardiac ryandodine receptors by means of open-state block, significantly reducing the Ca2+ mass spark without causing compensatory increases in the Ca2+ sarcolamatic reticulum content. Intravenous phlecainide reduces heart output and stroke volume in a temporary way. During chronic oral therapy, phlecainide has minimal effects on blood pressure,, and the LV (FV) ejection fraction remains unchanged, or decreases slightly, in patients with normal or almost normal ventricular function,,, however, phlecainide significantly reduces the stroke volume index and FEVI and increases pressures of the right capillary craving in patients with disease. According to current guidelines, phlecainide is indicated in patients with normal heart, hypertension, lower heart disease and good VV function, which is likely to apply to 80% of patients with paroxysmic FA (PAF) and 50% of patients with persistent FA, Generally speaking, the use of phlecainfenide of the 'real life' is low: the Eurocorromatic Survey of 1F indicates that about 17 and Before starting phlecainide treatment, patients should be checked for contraindications including structural heart disease, second or third grade AV block, left branch block of the package, right branch block (when associated with left hemiblock), unsustained asymptomatic ventricular tachycardia, cardigen shock, lower cardiac output (LVEF) In AF, oral phlecainide should be administered in a hospital setting with rhythm monitoring, starting with 50 mg of IADB and increased by 50 mg of IADB every 4 days until effectiveness is reached. After the administration of phlecainide heart rate should be monitored for at least 8 h, but doctors should review their local guide for mandatory hospitalization during titration. The recommended maximum dose is 300 mg/day. For patients who cannot receive high doses of standard oral phlecainide and those with kidney failure, a sustained release capsule can be used. To achieve control of the IC atrial flutter class, some doctors usually use digoxin or a beta blocker in addition to phlecainide. To achieve a faster effect in an emergency, a dose of phlecainide can be given as a slow injection of 1–2 mg/kg over 10 minutes, or in split doses, up to a maximum of 150 mg, while blood pressure is controlled. If they are not effective, a continuous infusion of phlecainide can be given to 1,2–1.5 mg/kg/h during the first hour and 0.12–0.25 mg/kg/h for later hours for no more than 24 hours. During the acute phase, the QRS is usually monitored continuously, but also measured with a 12-leaf ECG performed at the end of the bowl and 15 min, 30 min, 1, 2 and 3 h intervals. In patients receiving higher doses, monitoring of the ECG and plasma level is strongly recommended. The maximum dose accumulated over the first 24 h should not exceed 600 mg. Phlecainide can also be used for hospital patients and the elderly, although plasma cleansing is slower than in younger individuals. Clinical Performance Cardioversion Phlecainide is highly effective in the acute environment for AF cardioversion. In haemodynamically stable patients with acute-start AF (treated 48 h) and preserved LV function, flecainide restores SR by up to 95% of patients within 1h from the beginning of the infusion. A combined analysis of eight controlled trials randomized by the Health Research and Quality Agency (AHRQ) showed that acute treatment with phlecainide was associated with conversion rates of between 52 and 95% (Figure ). A randomized and randomized comparative study showed that SR was achieved in 90% of patients treated with phlecainide (2 mg/kg bolus, plus second 1 mg/kg pern if the first dose was not converted), compared to 72% of patients treated with propafenone and 64% of patients treated with amiodarone (P = 0.008). Although patients can also spontaneously convert SR, this usually takes much longer than with the active iv drug. In fact, the phlecainide significantly forced conversion to SR. Therefore, both hybrid and oral phlecainide can perform important functions by shortening the periods of symptomatic FA, thus limiting complaints. Proportion of subjects with successful pharmacological conversion (adapted from McNamara et al.). * Control treatment includes groups that receive placebo, Verapamil, diltiazem or digoxin; **, vertical lines represent 95% confidence intervals for the proportion of subjects with successful pharmacological conversion; +, n equals the number of tests that evaluate each comparison. Flecainide is also a safe and effective agent for termination of AF in patients with Wolff-Parkinson-White syndrome (WPW). Essentially, procainamide of iv is suggested as the frontline medication, but this is less effective to end the FF. By reducing the safety of driving on the accessory pathway, driving of flecainide blocks and slowing the ventricular rate. Phlecainide infusion during AF in patients with WPW is therefore extremely safe. In addition to slowing speed, flecainide eventually converts AF to SR. The effectiveness and safety of oral regimens (up to 300 mg in a single dose of load) and iv (up to 150 mg in 10 minutes) of phlecainide acetate have been clearly demonstrated (table ). In current guidelines, phlecainide (oral or iv) has received a class I, level A for cardioversion in AF. Approximately half of the patients who responded become 3 h of the oral dose or within 1 h of the initial infusion time., The oral dose of single-charge phlecainide has a conversion rate of 50–60% to 3h and 75–85% to 6–8h., An oral dose of propafenone load (600 mg) has also been proven effective for AF cardioversion, with conversion rates of around 72–76% to 6-8 h, although it takes more time, especially in the infusion of iv (3–6 average h). Table 1Prospective tests on the pharmacological conversion of oral fibrillation paroxysmal and persistent StudyPatient groupStudy type (level of evidence) Results Main results Capucci et al.62 patients with recent appearance AF (≤7 days), placebo versus amiodarone iv bolus followed by infusion or piscainide poRandomized blind trial Atrial flow with a 1:1 conduction (production of rapid ventricular rates) can occur immediately before conversion with a rate of 0.2 per cent, especially during exercise. A long Asian pause can also occur at the time of conversion. These are the main reasons for administering the first oral dose of charge under strict ECG and clinical control in a hospital setting. Subsequently, a single dose of bolus can be considered in an outpatient setting, after treatment has been considered safe, as a convenient method for cardioverting patients at home. This so-called "pill-in-the-pocket" approach has become a means of treating patients with paroxysmic or persistent symptomatic FA with an average rate of 70/min or greater. However, this strategy is only suitable for selected patients; the episode has to be of recent appearance (in 48 h) in a patient with normal QRS duration and good LV function, without nodal SA or AV dysfunction, bundle branch block, structural heart disease or Brugada syndrome. The advantage of the Pill approach in the pocket, despite the normally high rate of spontaneous conversion, is mainly related to the shorter time scale for the conversion associated with phlecainide, which can match a better QOL, although more evidence is required. Maintenance of sinus rhythm In PAF, phlecainide has been shown to significantly reduce the number of AF recurrences and lengthen the time between episodes.– A meta-analysis of 60 phlecainide studies showed that 65% of patients were sensitive to short-term treatment, and 49% in the long term, indicating that the clinical benefit of phlecainide to maintain SR is sustained. A literature analysis suggests that phlecainide may be more effective than other AADs to maintain the SR after cardioversion (Table), although no direct comparisons are available from head to head and these rates, taken with 12-letter ECG may be higher than those observed in the current monitoring guidelines. Table 2 Relapse rates for different antiarrhythmic drugs reported in the literaturea Average relapse rate (range)Studies (n)No drug69% (44–85)10Quinidine59% (46–89)11Disopyramide51% (46–56)3Propafenone61% (54–70)3Flecainide38% (19–513) Adapted from Levy et al. Phlecainide also reduces symptoms associated with AF; significantly more patients receiving phlecainide report palpitation suppression (P Clinical SafetyIn general, class 1C AADs are associated with specific risk factors for proarrhythmic events (Table). The electropharymacological effects dependent on use are increased at higher heart rates; therefore, electrophysiological effects are more marked in the atrium during the AF because the intrinsic atrial rate is so high. Therefore, the harmful effects (e.g. ventricular proarrhythmia, negative inotropy and AV block) are less risky in doses used to stop AF. The possible disadvantage of the use dependence is that, during the SR, the atrial (and ventricular) effects are lower, reducing the preventive effects. However, class 1C AADs suppress premature beats, suggesting that other mechanisms, such as deletion of automation (abnormal), can play a role. , Table 3 Ventricular proarrhythmia Risk Factors for Class Antiarrhythmic Medicines 1CRisk DrugsWide QRS (convide 120 ms), Brush ECG signUER LVEF, CHF Structural heart disease, CADHigh rate (use-dependent effect)Hypokalaemia High renal insufficiency (diac decreatinin ventricular depuration = 35 m2 CAST results posed major problems with AADs' safety to suppress arrhythmias or prevent arrhythmia recurrence., CAST results detered doctors from using phlecainide, even in patients without demonstrable cardiovascular disease. One of the most difficult problems is that patients can develop coronary disease, ischemia, and/or structural heart disease while receiving chronic phlecainide. Patients who are treated effectively but who have, for example, non-significant CAD as detected by CT angiogram may continue phlecainide but should be instructed about warning symptoms, including unexplained fatigue, new or increased chest pain, or syncope. Doctors should perform regular exercise and ECG tests, and patients should monitor their symptoms and report any problems. The most important thing is that background diseases, such as hypertension and coronary disease, should be addressed aggressively with preventive therapy once detected. Patients successfully treated with phlecainide but developing vascular disease may continue to treat phlecainide if these precautions are followed. When used in duly selected patients, phlecainide has shown a good safety profile, as shown by more than 25 years of cumulative experience with the drug throughout Europe and the United States. A recent systematic review determined the incidence of ventricular arrhythmias in patients treated with phlecainide to be The strengths of this meta-analysis are their amplitude and all the studies included were prospective; however, the quality of the studies differs markedly, and the follow-up was mostly relatively short. In spite of this, the findings remain valid; phlecainide appears to be safe for patients with supraventricular arrhythmias without detectable heart disease, and can contribute to the suppression of symptoms related to AF or SVT. The author concluded that the recommendation to perform intensive diagnostic tests to exclude associated cardiovascular disease, before beginning and during the treatment of phlecainide, is valid. Death attributable to phlecainide in meta-analysis was lower than expected in the general population (total mortality: 0.166%; 100-year mortality rate: 0.37). Compared to historical controls, the patient population of an AF study at Duke University (Durham, NC, USA) was much lower, although it contained a significant proportion of patients with extensive underlying heart disease. There was no evidence of increased or lower proarrhythmia events with phlecainide compared to compiled control drugs. Information on hospitalizations for the first time for AF was extracted from a national registry in Denmark between 1995 and 2004. Within this unselected cohort (n = 151 500) there was no association between antiarrhythmic treatment (flecainide, propafenone, sotalol or amiodarone) and any higher risk of death and showed that the proper selection of patients for AAD therapy did not increase mortality as suggested in other trials such as CAST. Annualized mortality rates (as per year per 100 years) were lower with class IC agents (flecainide: 2.54; propafenone: 4.25; sotalol: 5.29; and amiodarone: 7.42), and few deaths were observed within 30 days of AAD initiation, when the proarrhythmic effects of drugs are very likely. This study was limited by its non- random retrospective character, but the results are promising. In a study that evaluates the heart safety of the 200mg controlled acetate release formulation for the prevention of PAF, 4 out of 227 patients had a maximum QRS value √100 ms under treatment. Bradycardia (13.2%; n = 17/129) and ventricular extrasiles (10.6%; n = 11/104) were the most frequently identified proarritmic effects. Atrioventricular block (4.0%; n = 9/227), supraventricular tachycardia (2.2%; n = 5/227), bundle branch block (1.8%; n = 4/227), and AF (1.3%; n = 3/227) were the most common cardiac adverse events related to the drug. In this study, however, there was no comparison with the controls. It was concluded that the rate of adverse cardiac events was "consistent with literature data for patients with supraventricular tachyarrhythmia". The observation that the increase in QRS is the main cause of adverse effects related to phlecainide suggests that controlled release formulations can be safer than standard preparations. The place of phlecainide in the Flecainide atrial fibrillation is one of the first-line treatment recommendations to keep SR after cardioversion in the current guidelines. These guidelines advise that patients with recurrent PAF can benefit from the control of phlecainide rhythm, especially younger age groups with normal heart function. In the acute environment, phlecainide is recommended for PAF pharmacological cardioversion of no more than 7 days duration, and there are also strong tests that support the use of phlecainide before electrical cardioversion. A study found the use of pre-cardioversion phlecainide resulted in greater success in the first shocks compared to placebo (65 vs. 30%, respectively; P = 0.04). Another study concluded that "intravenous phlecainide reduces the threshold of atrial defibrillation in patients treated with low-energy internal atrial cardioversion that leads to a lower shock-induced discomfort. In addition, phlecainide may increase the procedure's success rate in patients with chronic persistent atrial fibrillation. The case for early treatment It is easy to underestimate the impact of AF. By the time the AF is confirmed, the refurbishment will be underway; the first documented episode can be only one of a series of unrecognized episodes. If not, the condition will become chronic through its own self-perpetuation mechanism. Clinicians who see many patients with AF are more aware of the impact of AF on patient well-being; patients with AF often do not fully appreciate the extent to which their QOL has been reduced until it has been restored. Atrial fibrillation is widely accepted as a condition of the elders; however, about half of the patients with PAF are When selecting the appropriate AAD, the treatment should be adapted to each patient; the often complex presentation of AF makes this task difficult. Flecainide has been on the market for 27 years, and its ability to reduce the symptoms of AF and provide long-term restoration of SR has been well documented. – However, the increase in mortality reported in CAST still deters many clinicians from using a 1C AAD class. This denies many new AF patients a valuable treatment option. The increase in mortality observed in patients treated with phlecainide in CAST is now considered to be caused by proarrhythmic events in older patients with a significant pre-existing cardiovascular comorbidity. Important treatment decisions should not be based on this unique study; there is now a body of evidence that supports the use of phlecainide, clearly demonstrating that in patients with AF without significant VV or CAD disease, the drug has a favorable safety profile with a low incidence of proarrhythmic and non-heartic adverse events. As a result of this evidence, phlecainide is now recommended as one of the first-line treatment options to restore and maintain SR in patients with AF under current ACC/AHA/ESC guidelines and updated ESC guidelines. ConclusionsThe atrial fibrillation is a 'fishing bomb'. The growing prevalence of AF can lead to an epidemic of heart disease associated with an important impact on QOL patients. Early detection and aggressive treatment can help break the vicious circle where the 'AF breeds AF'. For the past 25 years, phlecainide has been widely used worldwide. The abundance of experience and knowledge gathered during this period supports phlecainide as a safe and effective option to achieve and maintain SR in younger patients without co-existing structural heart disease. In addition, our increased understanding of the underlying physiophysiophysiological mechanisms of AF provides a sound rationality for early treatment with phlecainide to prevent long-term complications. Funding This review was supported by a Meda Pharmaceuticals educational grant. The representatives of Meda Pharmaceuticals had no role in the collection, analysis or interpretation of the information presented. The funding to pay the Open Access post for this article was provided by Meda Pharmaceuticals. The assistance of Patrick Wong in editing this document has been appreciated. Conflicts of interest: E.A. reports having received consulting fees from Meda Pharmaceuticals, Sanofi-Aventis, Pfizer and Bristol-Myers Squibb. H.J.C. reports having received funding of limited research and fees for Meda Pharmaceuticals speakers. A.G. reports having received 3M Pharmaceuticals speaker fees. ReferencesFormats: Share , 8600 Rockville Pike, Bethesda MD, 20894 USA
Log in to your account If you do not remember your password, you can reset it by entering your email address and by clicking the Reset Password button. You will receive an email containing a secure link to reset your password If the address matches a valid account, an email will be sent to __email___ with instructions to reset the password Anatomopathy with disease disorders Main adverse events Relative incidence Rare Recommendations for Flecainide Administration and Management Recommendations for Dosing and AdministrationIndicationDose rangeComments Acute conversion Oral200-300 mg, single administration2014 Guide AHA/ACC/HRS to practice management 2014; 64: e1-76,2016 ESC Guidelines for the management of atrial fibrillation developed in collaboration with eacts. Eur J Cardiothorac Surg. 2016; 50: e1-e88 First treatment under medical supervisionMaximum effect advance and conversion after 2-4 hours of BP monitor, ECG Intravenous1.5-2 mg/kg, maximum 150 mg, infused more than 10 min2016 ESC Guidelines for the management of atrial fibrillation developed in collaboration with eacts. Eur J Cardiothorac Surg. 2016; 50: e1-e88 Anticipate the maximum effect and conversion at the end of the BP infusion Monitor near, ECGChronic prevention50-200 mg bidStart a 100 mg offer, adjust after 4 days. ECG Monitor, check the duration of QRS before dose escalation Concomitant therapy with beta block AgentsConclusionsDisclosuresReferencesArticle InfoPublication HistoryOcidentificationDOI: Copyright User License Permitted For Non-commercial Purposes: Not AllowedScienceDirectPlease enter a term before submitting your search. We use cookies to help provide and improve our personalized service and content and ads. By continuing you accept the . Copyright © 2021 Elsevier Inc. except for certain content provided by third parties. The content of this site is intended for health professionals.

The Utility of Beta-Blockers Immediately Prior to Alcohol Consumption in Order to Prevent Atrial Fibrillation | EP Lab Digest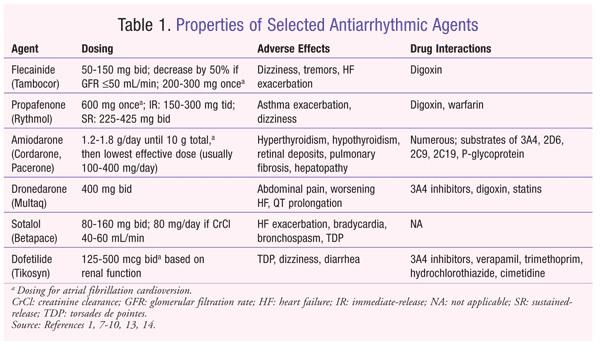
Antiarrhythmic Therapy for Atrial Fibrillation
Use of Flecainide for the Treatment of Atrial Fibrillation - American Journal of Cardiology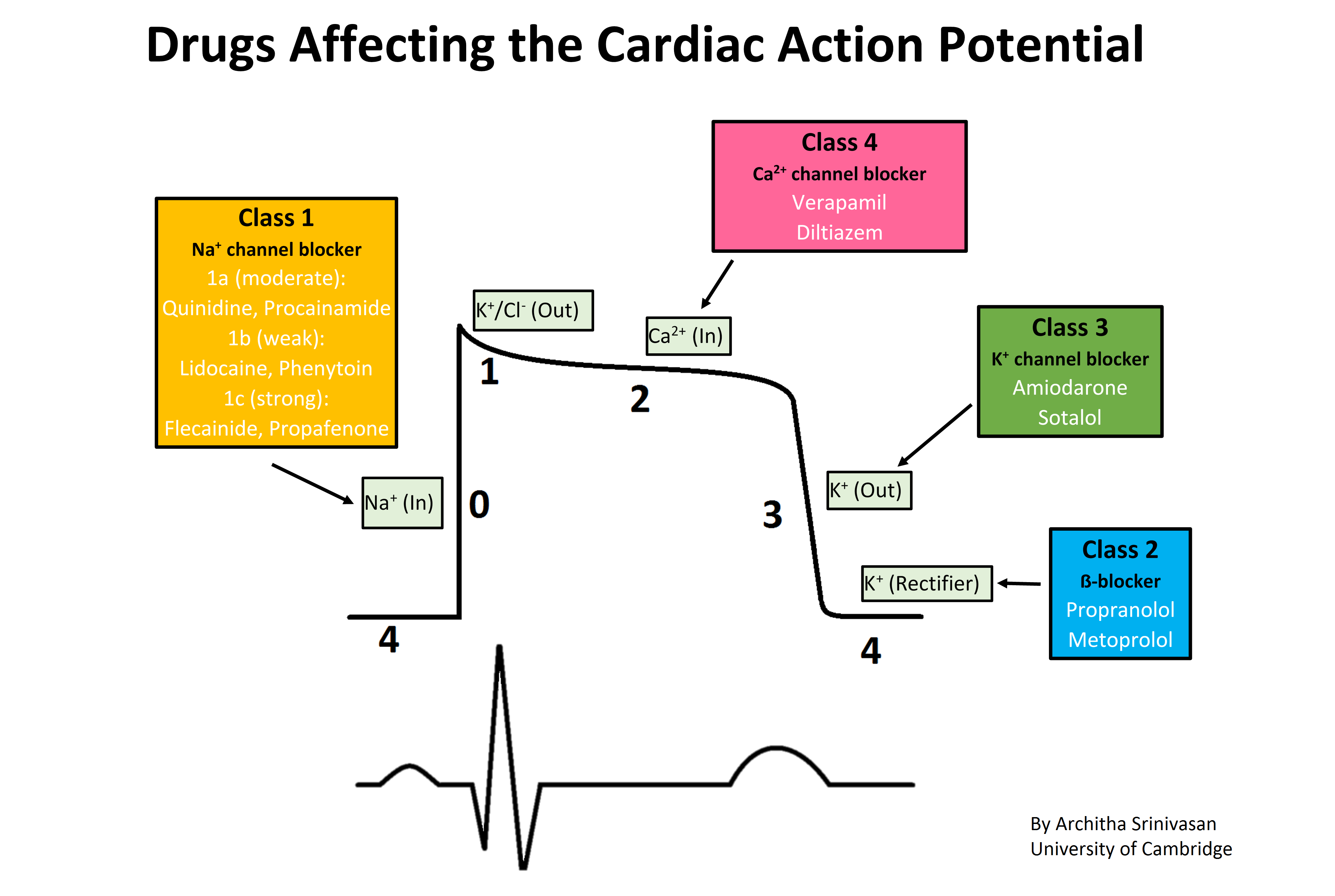
Antiarrhythmic agent - Wikipedia
EP Summit 2015: Practical Pearls in AAD Selection and Management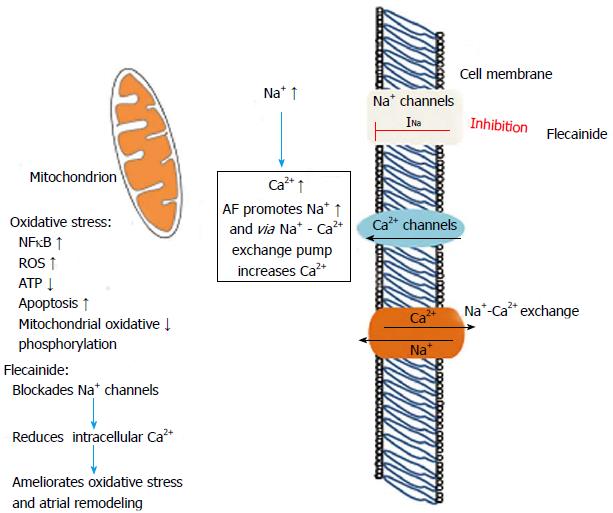
Flecainide: Current status and perspectives in arrhythmia management
Antiarrhythmic mechanisms of beta blocker therapy - ScienceDirect
Anyone try flecainide? Failed beta blockers and now failing high dose calcium channel blockers. : PVCs
Abstract 12009: A Prospective Randomized Placebo-Controlled Crossover Trial of Flecainide for Catecholaminergic Polymorphic Ventricular Tachycardia | Circulation
Diagnosis and Management of Common Types of Supraventricular Tachycardia - American Family Physician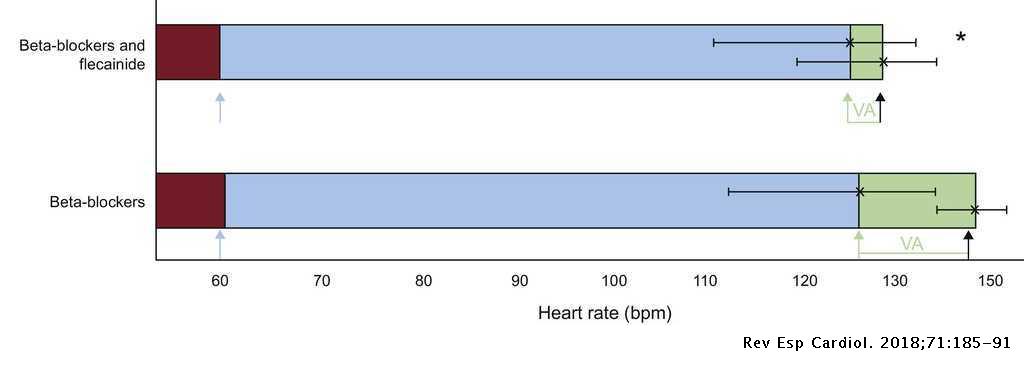
Flecainide Reduces Ventricular Arrhythmias in Patients With Genotype RyR2-positive Catecholaminergic Polymorphic Ventricular Tachycardia | Revista Española de Cardiología (English Edition)
Use of Flecainide for the Treatment of Atrial Fibrillation - American Journal of Cardiology
Therapies recommended for long-term treatment of patients with... | Download Table
Safety and efficacy of flecainide in association with beta-blockers in arrhythmogenic right ventricular cardiomyopathy - ScienceDirect
Flecainide Uses, Side Effects & Warnings - Drugs.com
CAST beta blocker AA drugs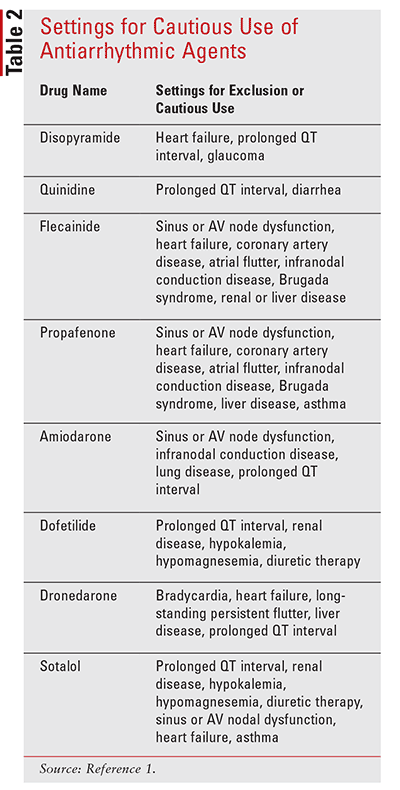
Antiarrhythmic Treatment in Atrial Fibrillation
Flecainide Acetate Tablets USP
Classification and choice of antiarrhythmic therapies - Barton - 2020 - Prescriber - Wiley Online Library
Flecainide: A Pill In The Pocket For AFib — Dr. AFib™
Flecainide therapy suppresses exercise-induced ventricular arrhythmias in patients with CASQ2-associated catecholaminergic polym
RYR2 Channel Inhibition Is the Principal Mechanism of Flecainide Action in CPVT | Circulation Research
Recommendations for treatment of focal atrial tachycardias | Download Table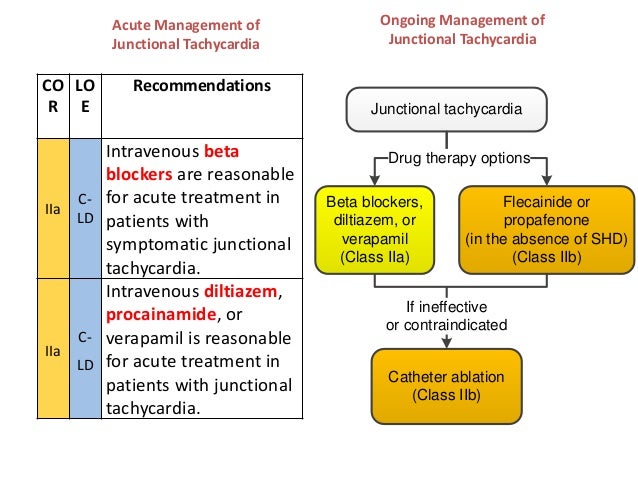
2015 svt guideline final 19.10.2015
Flecainide normalizes Ca2+ spark parameters in CPVT iPSC-CMs during... | Download Scientific Diagram
A Case of Flecainideâ•'Induced Hyponatremia
Flecainide - Wikipedia![A]()
A "Pill-in-the-Pocket" Approach to Paroxysmal Atrial Fibrillation — NUEM Blog![Outpatient Treatment of Recent-Onset Atrial Fibrillation with the]()
Outpatient Treatment of Recent-Onset Atrial Fibrillation with the "Pill-in-the-Pocket" Approach | NEJM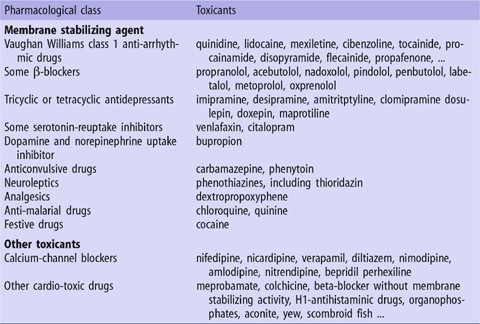
Extracorporeal Life-Support for Acute Drug-induced Cardiac Toxicity | SpringerLink
Guidance on Short‐Term Management of Atrial Fibrillation in Coronavirus Disease 2019 | Journal of the American Heart Association
Los Angeles Echo Society - Anti Arrhythmics Here's some more EP (Electrophysiology) knowledge for you- Antiarrhythmics! Often the bane of many people's existences is the ability to remember what class of anti
Enlightened Medical Management of Atrial Fibrillation, Part II: The Pill In The Pocket Approach – The Skeptical Cardiologist
Flecainide Uses, Side Effects & Warnings - Drugs.com
New Antiarrhythmic Drugs: Tocainide, Mexiletine, Flecainide, Encainide, and Amiodarone - Mayo Clinic Proceedings
Pin by BOYA RAMU on Doctor Ramu | Potassium channel, Sodium channel, Metoprolol
Multiple targets for flecainide action: implications for cardiac arrhythmogenesis - Salvage - 2018 - British Journal of Pharmacology - Wiley Online Library
Comparative haemodynamic effects of intravenous flecainide in patients with and without heart failure and with and without beta-
Flecainide - wikidoc
Unmasking of Sick Sinus Syndrome Using Flecainide in a Patient with SCN5a Mutation and Overlap Syndrome - Heart, Lung and Circulation
 PDF) Flecainide–metoprolol combination reduces atrial fibrillation clinical recurrences and improves tolerability at 1-year follow-up in persistent symptomatic atrial fibrillation
PDF) Flecainide–metoprolol combination reduces atrial fibrillation clinical recurrences and improves tolerability at 1-year follow-up in persistent symptomatic atrial fibrillation


































Posting Komentar untuk "is flecainide a beta blocker"